In July 2017 the MHRA approved the reclassification of atovaquone 250mg/proguanil 100mg from a POM to a P medicine for chemoprophylaxis of P.falciparum* in adults (18yrs and over) travelling for up to a maximum of 12 weeks in a malaria endemic area. The change became effective in September 2017 with Maloff Protect now available to purchase through pharmacy
*In 2016 P. falciparum represented 80% of malaria cases reported

Keys actions to take when supplying Maloff Protect :
- Check country being visited
- Check traveller is suitable for Maloff Protect
- Calculate the number of tablets required
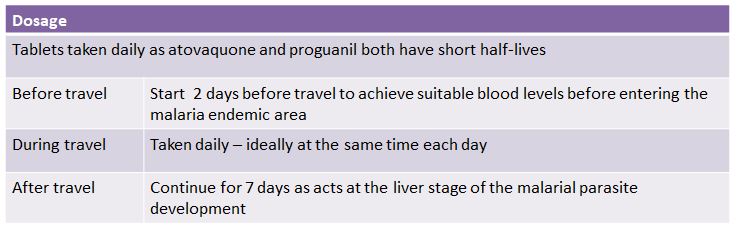
- Advise the traveller how to take the tablets (see table 1.)
- Advise of potential side effects
- Provide practice advice on bite prevention
- Advise of symptoms of malaria and importance of early diagnosis
Visit Maloff Protect Pharmacy Zone for supporting materials by clicking here.
It is advisable for travellers to have a full travel health consultation including vaccinations
Numark can support you to offer a full travel clinic and vaccination service – for more information visit Numarknet by clicking here.
Table 1.