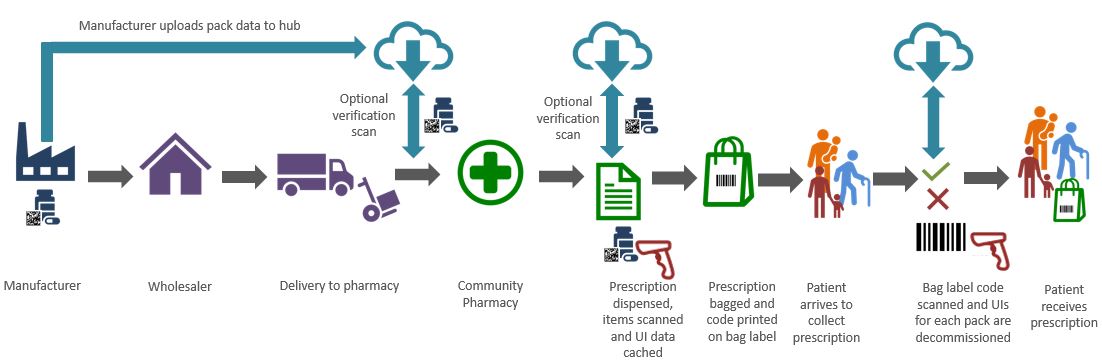
FMD will require community pharmacy teams to include additional steps within their dispensing processes, these steps must be reflected in revised pharmacy Standard Operating Procedures.
All relevant prescription medicines will need to be authenticated and verified prior to supply to the patient. To authenticate and verify a medicine pharmacy teams should:
- Visually check the Anti-Tamper Device (ATD) to confirm it is intact
- Scan the Unique Identifier (UI) to decommission the product
Decommissioning will change the status of the medicine pack on the NMVS from “active” to “inactive”. This will prevent people who falsify medicines from duplicating the UI as any attempt to decommission the same UI will generate an alert message showing the medicine pack has already been decommissioned and may be falsified.
FMD requires that decommissioning takes place “at the time of supply” to the patient (see the section later in this article discussing the 10 day rule for more information).