Despite pressurised doses and tissue compression there remains a concern that certain patient groups may receive adrenaline subcutaneously rather than intramuscularly. This is because the length of the needle may not be long enough to penetrate the muscle fascia and allow adrenaline to reach the muscle tissue. This could result in a reduced response to treatment
Patient groups at risk are female patients and young children. The risk in children is associated with shorter needles being used in lower dose AAI.
Where there is a possibility that patients may have a larger than average STMD it would be advisable to use an AAI with a longer needle length. Currently Emerade is the only available AAI with a needle length exceeding 20mm. For pharmacists delivering vaccination services, without the foresight of knowing the patient's likely STMD, it would seem prudent to stock devices with longer needles
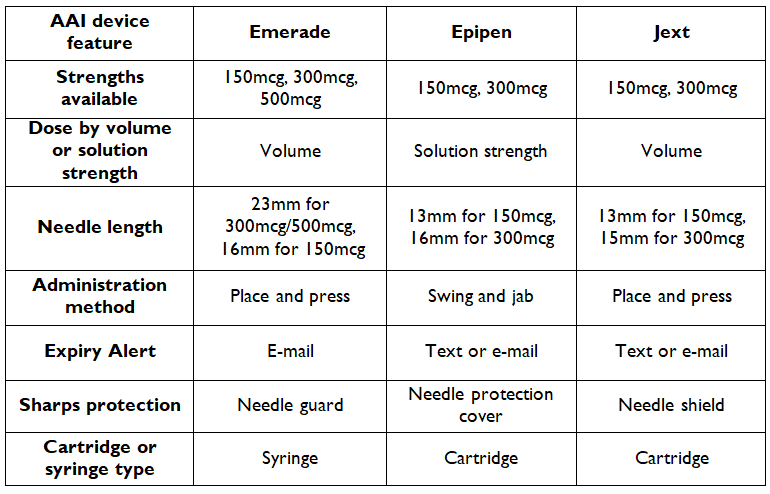
The following table compares the key features of the individual AAI: