Nearly 1.2 million prescriptions for fentanyl products were dispensed in the community in 2017.4
The term "opioid" refers to morphine-like synthetic or endogenous compounds. Therapeutically, the synthetic opioids are reserved for the treatment of moderate to severe pain. This is because of the undesirable adverse effects of nausea, vomiting, constipation and drowsiness, together with the incidence of respiratory depression associated with higher doses.
Fentanyl is a phenylpiperidine opioid agonist that is licensed for the treatment of malignant and non-malignant chronic intractable pain. The analgesic effect of fentanyl is about 100 times more potent than morphine although it has a much shorter duration of action.7
Transdermal fentanyl is not routinely recommended as first line treatment for chronic pain. NICE guidance states that morphine should be the first line opiate for use in pain relief, but for patients unable to tolerate oral opiates transdermal fentanyl is considered a suitable treatment option.5
Situations where transdermal fentanyl would be appropriate would include patients who have stable pain but experience difficulty swallowing medicines, patients with renal failure, persistent nausea and vomiting, gastro-intestinal obstruction, poor compliance with oral medication or suffer intolerable side effects from oral opiates. The combination of consistent plasma levels provided by transdermal administration and the different affinity for the opiate receptors of fentanyl reduces the incidence of opiate related side effects. For example fentanyl has a lower affinity for the opiate receptors in the gut wall than morphine and consequently has a reduced constipating effect than morphine.
Adverse effects
Potential adverse effects associated with transdermal fentanyl include insomnia, somnolence, dizziness and headache, nausea, vomiting and constipation. These adverse effects are considered very common and can affect more than one in ten patients.6 Constipation, which is a commonly reported side effect of oral morphine, is less troublesome in patients using fentanyl, and patients transferred to fentanyl from morphine should have their laxative doses reduced by approximately half.7
Fentanyl and morphine equivalence
The strength of fentanyl patch initially prescribed for a patient will be determined by their previous exposure to opioids and any consequent development of tolerance. Opioid naïve patients should be initiated with a transdermal fentanyl dose no higher than 25mcg/hour to avoid the development of respiratory depression. Patients who are currently being treated with opioids should have their current daily opioid dose converted to an equianalgesic oral morphine dose and then convert this to an equipotent fentanyl patch. In both situations the patient's response should be monitored closely for adequate analgesia and also signs of opiate toxicity, in particular respiratory depression.
Tables to facilitate conversion are included in the summary of product characteristics (SPC) for each brand of fentanyl patch. Manufacturers list a wide range of oral morphine doses corresponding to each fentanyl patch resulting in a wide range of conversion ratios. The Palliative Care Formulary (PCF) endorses a conversion ratio of oral morphine: transdermal fentanyl of 100:1 as safe and effective.7 NICE advises "use caution when calculating opioid equivalence for transdermal patches: A transdermal fentanyl 12mcg patch equates to approximately 45mg oral morphine daily".5
The following table gives a guideline for converting oral morphine to transdermal fentanyl (based on dose ratio 100:1) but it should be used in conjunction with the SPC for the fentanyl patch being used.
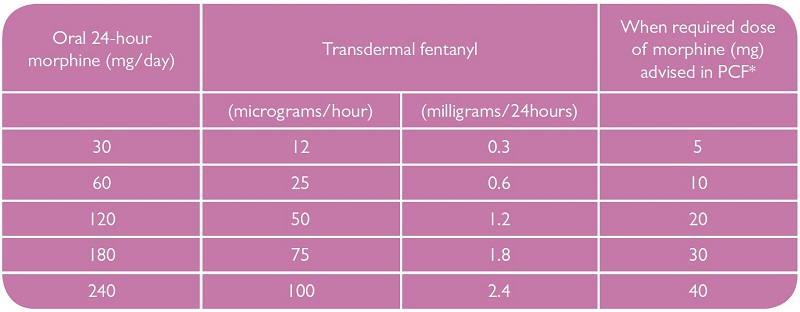
*Using a traditional 1/6 of total daily dose as when required dose.8
In situations where the oral 24 hour morphine dose does not directly correspond to an equivalent fentanyl patch, a clinical decision should be made over the most appropriate strength of patch to initiate treatment. The patient's response must be monitored closely for on-going pain or opiate side effects.
In both opioid naïve and tolerant patients, it is important to monitor the patient's response closely. Transdermal administration results in a delay in analgesic effect with the maximum analgesic effect not being seen until the patch has been worn for 24 hours. This delay in initial response may require concurrent administration of a reducing dose of oral or parenteral analgesic until the full transdermal effect is obtained.
Patients transferred from oral morphine to transdermal fentanyl should have their morphine dose continued as follows8:
- If taking immediate release morphine every four hours, continue with regular morphine doses for 12 hours after application of the fentanyl patch.
- If taking 12 hour sustained release morphine, give the last dose at the same time as applying the fentanyl patch.
- If taking 24 hour sustained release morphine, apply the first patch twelve hours after the final morphine dose.
An evaluation of the maximum analgesic efficacy of the fentanyl patch should not be made until the patch has been worn for 24 hours.
Patients transferred from oral morphine to transdermal fentanyl may experience withdrawal symptoms despite adequate pain relief. Opiate withdrawal symptoms can include restlessness, nausea, sweating, diarrhoea and colic. The proposed reason for this withdrawal effect is differences in the relative affinities of fentanyl and morphine at the peripheral and central mu opioid receptors. Patients should be counselled on the possibility of these effects and where necessary these symptoms can be controlled with rescue doses of morphine until they resolve after a few days.7
