Vaccine Changes
2025-2026 is seeing a change in influenza vaccines from quadvalent to trivalent. This is due to the World Health Organisation concluding that the B/Yamagata lineages are no longer circulating and should no longer be included in vaccines. The Live-Attenuated Influenza Vaccine has already shifted to a trivalent format and for inactivated vaccines JCVI prefers trivalent over quadvalent formulations (where available).
Make sure you are familiar with who should have which vaccine. You can find full details in the National flu immunisation programme 2025-2026 letter.
Check which vaccines are available for the 2025/2026 season here JCVI statement on influenza vaccines for 2025 to 2026.
You can find ordering details for odering flu vaccinations from Numark here.
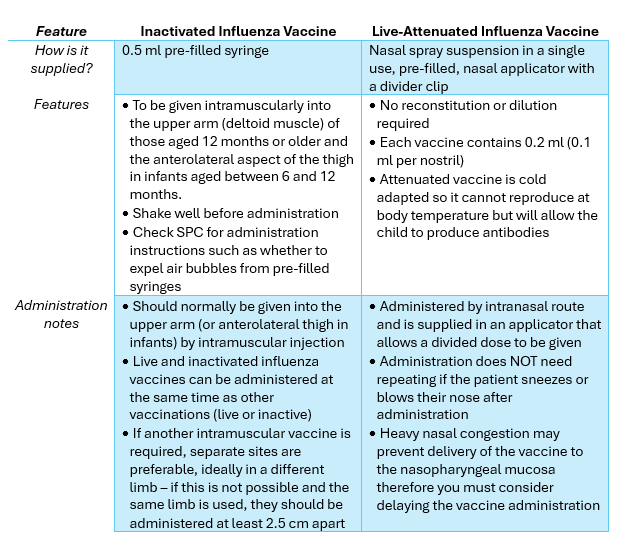
Vaccine Overviews
NOTE: Children that are between 6 months and 9 years old that are in a clinical risk group (see 2025/2026 Patients) and have not previously been vaccinated against influenza will require a second dose.
Potential Side Effects
Common Adverse reactions
These are the commonly reported symptoms after intramuscular injection. These symtoms usually disappear within 1-2 days without treatment
|
|
|
|
|
|
|
|
|
|
These are the commonly reported side effects following administration of the LAIV:
- Nasal congestion/runny nose
- Reduced appetite
- Weakness
- Headache
There are some rare side effects that are usally immediate reactions. These include urticaria, angio-oedema, bronchospasm and anaphylaxis.