Chickenpox Vaccine
Treating Chickenpox is, of course, a very good thing but prevention is always going to be better than cure.
Luckily there is a vaccine available. The recommended two doses of the vaccine are estimated to offer 98% protection from chickenpox in children and 75% protection in adolescents and adults. Although rarer than an unvaccinated person if a person gets chickenpox after being vaccinated, they will usually have milder symptoms than someone who has not been vaccinated.
The website NHS Chickenpox Vaccine reminds us that there are currently two Chickenpox vaccines available in the UK with some useful information regarding their administration.
- Both require reconstitution and should be given as a 0.5ml dose.
- Both can be administered by either subcutaneous or intramuscular injection.
- The course can be completed effectively with the different vaccines (Because they work in the same way. Covered further down this page)
- Children (who require vaccination from one year of age or older) and adults should receive their two doses of varicella vaccine, four to eight weeks apart (and certainly not less than four weeks apart).
- In both age groups, most of the breakthrough infections are modified and vaccinated individuals who contract varicella have fewer lesions and less systemic upset than unvaccinated individuals.
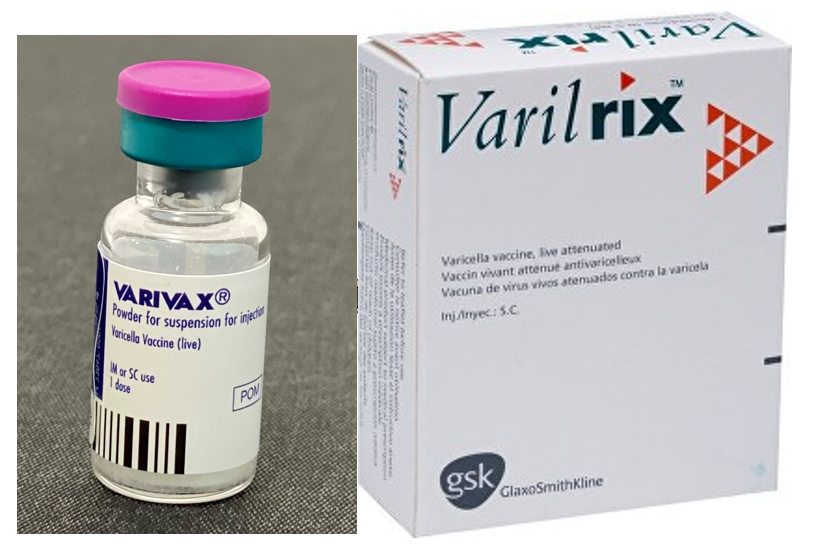
| Supplier | Name of Product | Vaccination Type |
| MSD | Varivax® | Powder and solvent for suspension for injection. Oka/Merck strain (live, attenuated) |
| GSK | Varilrix® | Powder and solvent for solution for injection. Oka strain (live, attenuated) |
Mode of action
Both vaccines contain a weakened form the living varicella virus. This strain of live virus causes either mild or no symptoms of infection. It works by causing your body to produce its own protection against chickenpox. It does this by making disease-fighting substances called antibodies to fight the varicella virus. If a vaccinated person comes into contact with the live virus, the body is usually ready to destroy it.
Storage
Vaccines may lose their effectiveness if they’re kept outside the recommended temperature range, and this cannot be reversed. Storage requirements are listed within each vaccine’s Summary of Product Characteristics (SPC), so you should refer to these to ensure vaccines are kept in the correct environment. Vaccines can also be sensitive to light exposure, so the SPC will outline storage requirements to protect them from light.
Vaccine stocks should be monitored regularly to make sure there is approximately two to four weeks' supply of each vaccine, thereby reducing wastage and reducing the risk of administering an out-of-date vaccine. You should keep track of expiry dates and label and dispose of any out-of-date stock according to local policies. Vaccines must never be used past their expiry date.
The cold chain for vaccines refers to the cold temperature conditions required for their transportation and storage. This is usually at a refrigerator temperature of +2°C to +8°C. Most vaccines should not be frozen, although certain vaccines require storage at temperatures below 0°C.
Always consult the manufacturer's summary of product characteristics (SPC) for temperature requirements.
Before vaccination check:
- The product and dose are correct
- The expiry date
- Colour and composition of the vaccine matches the description in the product’s SPC
- There are no contraindications
- Patient is fully informed about the vaccines and has given consent
- Patient is aware of possible adverse reactions
If the patient is receiving more than one injection in the same appointment, it should be given at separate sites and preferably in different limbs. If it has to be in the same limb, they should be given 2.5cm apart. Ensure to record the site of their injection.
After vaccination:
- Record information of vaccine name, product name, batch number and expiry date
- Record dose administered
- Which limb used
- Date of vaccination
- Name and signature of the vaccinator
- You could provide a vaccines record with details of vaccine received
- Suggest to inform their GP so can be added to their medical records
- If they have confirmed that they may faint – you could observe them a little longer after they have had their vaccine.
Side effects of the chickenpox vaccine
Most side effects of the chickenpox vaccine are mild and do not last long.
They can include:
- Swelling or pain where the injection was given
- A high temperature
- A rash in the area where the injection was given or more widespread – it usually develops within 1 month of vaccination
More serious side effects, such as a severe allergic reaction, are very rare. The person who vaccinates you will be trained to deal with allergic reactions and treat them immediately.
Eligibility
See the full service inclusion criteria stipulated in the Patient Group Direction (PGD) you are using however NHS Chickenpox Vaccine informs us that the chickenpox vaccination service may be suitable for adults and children who:
- Haven’t already had the vaccine
- Haven’t had chickenpox before
- are at least two years old, and less than 65 years old
- Are not pregnant or breastfeeding
- Are not immunocompromised (a weakened immune system)
- Are health workers who have patient contact
- Are not eligible for an NHS vaccination but want to help protect themselves and their family from the disease
- Who have contact with immunocompromised patients, particularly where continuing close contact is unavoidable
- Haven’t had an allergic reaction to a vaccine before
- Are laboratory staff
The Chickenpox vaccine is not currently part of the routine childhood vaccination schedule. However in 2023 the government (Joint Committee on Vaccination and Immunisation) JCVI Chickenpox also recommended that the vaccine be offered to all children in 2 doses, at 12 and 18 months of age.
Chickenpox Vaccine and Shingles Vaccine are not the same thing.